The Group

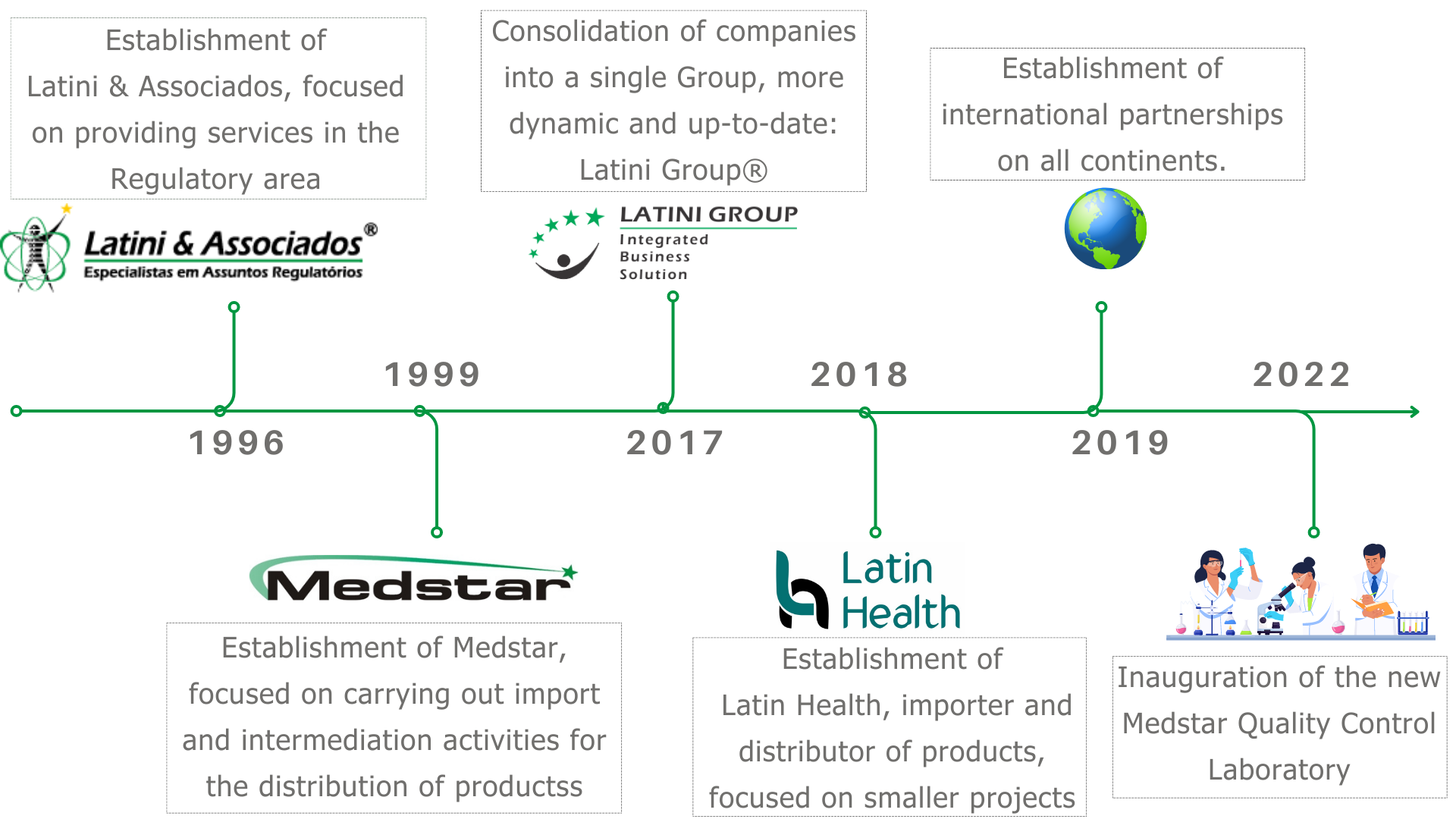
Aiming at a new value proposition, in 2017, Latini's brands consolidated into a single, more dynamic and new brand: Latini Group®.
Latini Group® is the consolidation of a group of reference companies with more than 27 years of experience and excellence in Regulatory Affairs, offering integrated and customized solutions for the needs of each client (One Stop Shop). Providing complete management of technical and legal responsibility for products to be registered in Brazil, in addition to full logistical support.
Our expertise allows us to serve the market by minimizing risks for commercial entry through quality regulatory standards and by highly trained professionals in tune with technological innovations supported by research, permanent publications, joint work with class institutions and important national and international partnerships.
Our pillars of action are:
- Regulatory Affairs;
- Compulsory certifications;
- Compliance in the Health area;
- International Trade (Import/Export/Distribution/Local Inventory Management);
- Hosting of product registration (ANVISA).
Count on an experienced and consolidated partner in the area. Count on Latini Group!
Learn more about each company of the Group:

Regulatory Affairs are our expertise!
Latini & Associados® is a company specialized in Regulatory Affairs in Brazil, with strategic partnerships in Latin America and other continents.
Founded in 1996, it is part of the Latini Group® and a world reference, with thousands of approved processes. It has a specialized multidisciplinary technical team, allowing the reduction of time in the elaboration of processes, with gains in efficiency, cost and allocation of personnel.
Company certified in accordance with the ISO9001 Standard by the Notified Body TÜV NORD Brasil.
![]()
Complete Regulatory Management and Logistics!
Medstar® was founded in 1999, being part of the Latini Group®, duly licensed to provide the Registration, Hosting, Import, Export, Distribution and Sale of products controlled by the Sanitary Surveillance Agency, such as medicines, pharmaceutical raw materials, sanitizers, cosmetics, food supplements and medical devices.
Medstar® has all Federal Licenses, Certificates and Operating Authorizations to guarantee the necessary quality for international trade processes and product registration management for companies not established in Brazil.

Opportunity for foreign companies to enter the regulated market in Brazil!
Latin Health® is an Importer and Distributor of Medical Device Products and Cosmetics, allowing local and foreign companies to quickly enter the regulated market, with reduced costs and deadlines.
As part of the Latini Group®, it presents itself as a low-cost company and an excellent option for starting a business in the Brazilian market.